GENE THERAPY & Clinical Trials TO TREAT VISION LOSS
When it comes to innovative therapies for treating eye diseases no approach has gained more attention than gene therapy. And rightfully so. The first gene therapy for any eye disease (Luxturna®) was approved in the United States (US) in 2017, for people with RPE65 mutations causing Leber congenital amaurosis (LCA) or retinitis pigmentosa (RP). Currently, there are dozens of gene therapy clinical trials underway with even more gene therapies being researched and developed. Keep reading to learn about different types of gene therapy, who may be eligible, and for a list of ongoing gene therapy clinical trials.
What is gene therapy and how does it work?
Different cells in the body have different functions. Lung cells help absorb oxygen, muscle cells help us move, and retinal cells absorb and transmit light signals to form images. But how does each different cell know what to do? It all comes down to our genes. Genes, which are made of DNA, are the cell’s instruction manual, providing information on what molecules the cell should make and how the cell should act.
The purpose of gene therapy is to try and change how the cell is acting by putting in a new gene or replacing a broken gene. Gene therapy is at the forefront of new treatments for inherited retinal diseases (IRDs) like retinitis pigmentosa (RP) or Stargardt disease, which are caused by a broken or mutated gene. Gene therapy is also being explored for other types of vision loss, including age-related macular degeneration (AMD), diabetic macular edema (DME), and corneal eye disease. Here are some of the most common types of gene therapy:
Gene Replacement
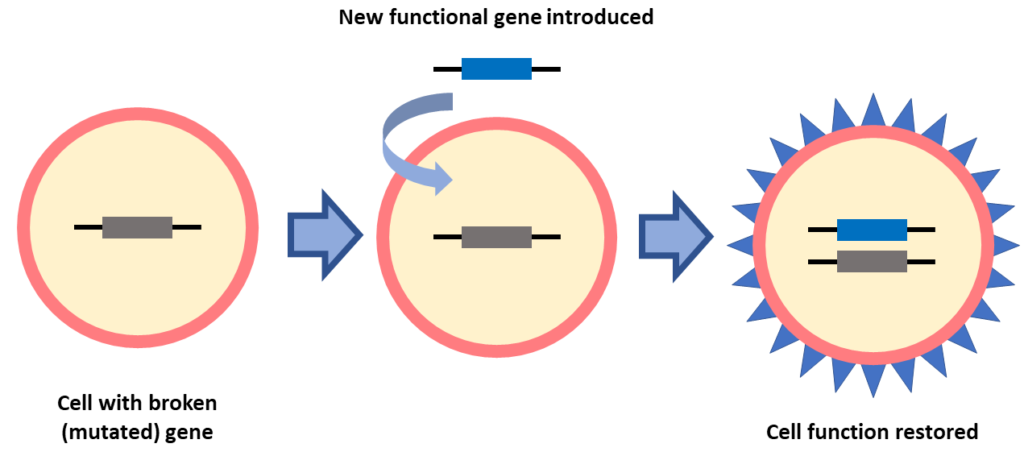
In gene replacement a new gene is introduced into a cell to replace a broken gene. This form of gene therapy is being used for many IRDs and is the basis of the first approved gene therapy called Luxturna. Gene replacement might be a good option for people who know their gene mutation.
Gene editing
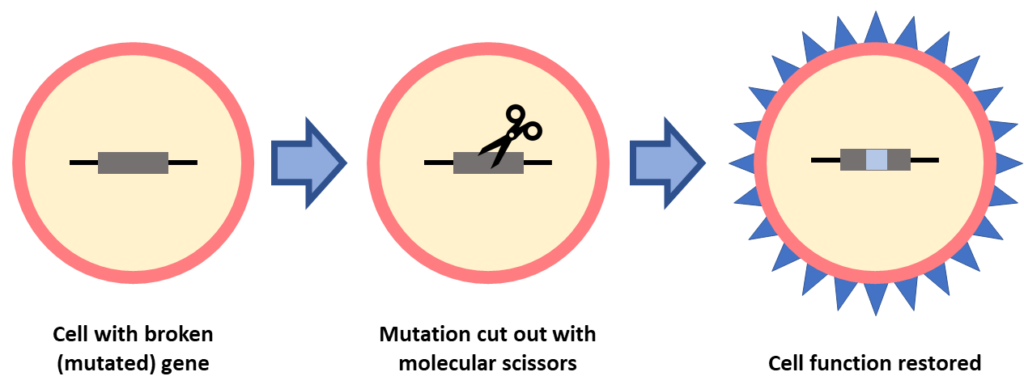
This is also a gene-specific treatment, where the gene mutation must be known. In gene editing, molecular scissors are used to “edit” a broken gene by cutting out the mutation. This form of gene therapy is being studied as a treatment for IRDs, including RP, LCA and Usher syndrome.
Gene therapy that stops cell death
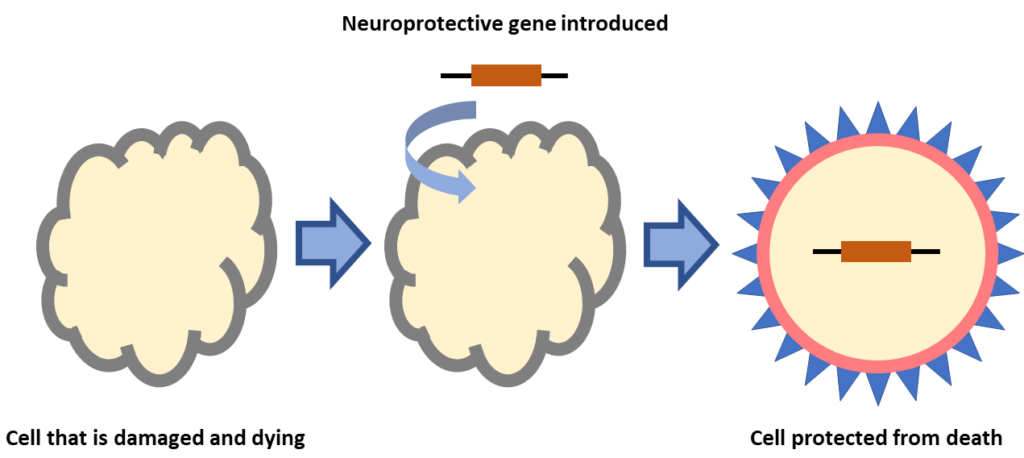
Retinal diseases like IRDs, AMD, and DME have different genetic and environmental causes. In all cases vision loss occurs when retinal cells that help sense and send light signals die. Scientists are developing gene therapies that are “neuroprotective” meaning that genes are introduced to retinal cells that stop them from dying. This type of gene therapy is not gene-specific so a single treatment could be used for, IRDs caused by different types of mutations, IRDs where the gene mutation has not been identified, or diseases like AMD which might not have a genetic cause.
Gene therapy to replace anti-VEGF injections
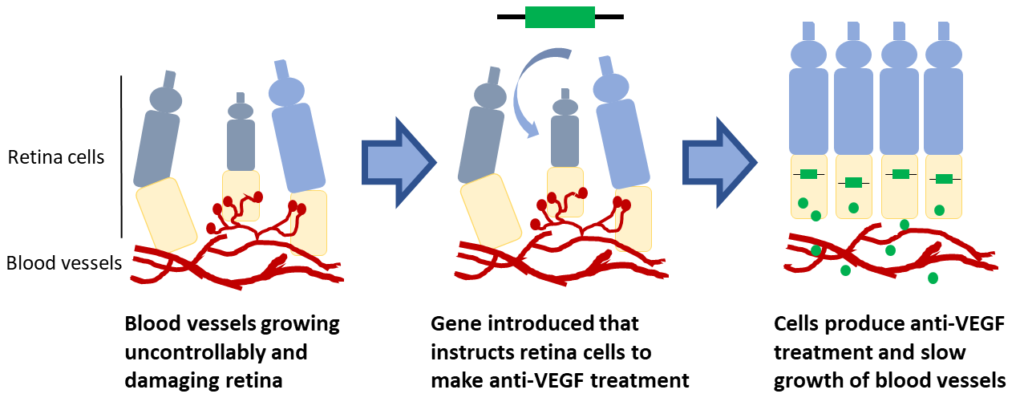
Anti-VEGF injections are the most common treatment for AMD and DME. In both AMD and DME, vision loss is caused when blood vessels in the retina grow uncontrollably and leak fluid into the eye. Anti-VEGF treatments work by slowing the growth of blood vessels. Clinical trials are testing if genes can be introduced into retinal cells which instruct cells to produce their own anti-VEGF. These trials are trying to turn retinal cells into little anti-VEGF factories with the goal of reducing the need for frequent injections.
Some of the challenges
Gene therapy has shown a lot of promise to provide treatments for a variety of eye diseases. There are some challenges and important considerations for people seeking this treatment. Currently, the gene therapies that are showing the most promise are gene specific and for IRDs like RP or LCA.
One of the challenges with gene specific therapies is that it can take a lot of time and money to make a gene therapy for each different gene or mutation. And this type of treatment only works for people with the exact gene mutation for which it is designed.
Another challenge is the stage of disease. Gene therapy approaches will only work if there are enough healthy retinal cells remaining into which the gene therapy can be introduced. With few healthy retinal cells, other approaches like stem cell treatment or artificial retinas may be more appropriate.
Luxturna: the first gene therapy for an IRD
Luxturna is the first approved treatment for an IRD in Canada. It is also available in the US and Europe. Luxturna is a gene therapy for individuals with RP or LCA who have mutations in both copies of the RPE65 gene. The therapy is not a cure but can improve vision and may slow the progression of vision loss.
Gene therapy clinical trials
There are many new gene therapies being studied for different types of vision loss, many of which are being tested in patients in clinical trials. Visit our Clinical Trials Page to find information about clinical trials happening all over the world. Each trial has very specific eligibility criteria. If you are interested in participating in a trial talk to your eye doctor.
For more information about gene therapy trials, contact our Health Information Line at 1.888.626.2995 or by email at healthinfo@fightingblindness.ca
This resource page is part of FBC’s “Clinical Trials Corner Series”.
Learn more
- Looking into the genetics of inherited retinal diseases with Dr. Rob Koenekoop: In this article, Dr. Rob Koenekoop discusses how his research into the genetics of inherited retinal diseases (IRDs) has led to new treatments and how patients can participate.
Join the Fight!
Learn how your support is helping to bring a future without blindness into focus! Be the first to learn about the latest breakthroughs in vision research and events in your community by subscribing to our e-newsletter that lands in inboxes the beginning of each month.

